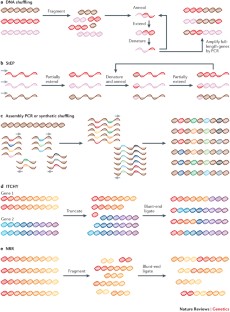
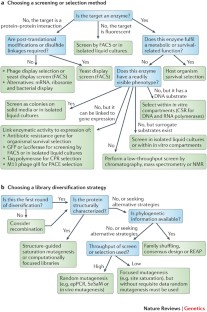
Methods for the directed evolution of proteins
Directed evolution has proved to be an effective strategy for improving or altering the activity of biomolecules for industrial, research and therapeutic applications. The evolution of proteins in the laboratory requires methods for generating genetic diversity and for identifying protein variants with desired properties. This Review describes some of the tools used to diversify genes, as well as informative examples of screening and selection methods that identify or isolate evolved proteins. We highlight recent cases in which directed evolution generated enzymatic activities and substrate specificities not known to exist in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
In vivo hypermutation and continuous evolution
Article 19 May 2022

The developing toolkit of continuous directed evolution
Article 22 May 2020
Directed evolution in mammalian cells
Article 07 April 2021
References
- Wright, S. I. et al. The effects of artificial selection on the maize genome. Science308, 1310–1314 (2005). ArticleCASPubMedGoogle Scholar
- Driscoll, C. A., Macdonald, D. W. & O'Brien, S. J. From wild animals to domestic pets, an evolutionary view of domestication. Proc. Natl Acad. Sci. USA106 (Suppl. 1), 9971–9978 (2009). ArticleCASPubMedGoogle Scholar
- Umeno, D., Tobias, A. V. & Arnold, F. H. Diversifying carotenoid biosynthetic pathways by directed evolution. Microbiol. Mol. Biol. Rev.69, 51–78 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Atsumi, S. & Liao, J. C. Directed evolution of Methanococcus jannaschii citramalate synthase for biosynthesis of 1-propanol and 1-butanol by Escherichia coli. Appl. Environ. Microbiol.74, 7802–7808 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wang, H. H. et al. Programming cells by multiplex genome engineering and accelerated evolution. Nature460, 894–898 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Zhang, Y. X. et al. Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature415, 644–646 (2002). ArticleCASPubMedGoogle Scholar
- Alper, H., Moxley, J., Nevoigt, E., Fink, G. R. & Stephanopoulos, G. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science314, 1565–1568 (2006). ArticleCASPubMedGoogle Scholar
- Coelho, P. S., Brustad, E. M., Kannan, A. & Arnold, F. H. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science339, 307–310 (2013). ArticleCASPubMedGoogle Scholar
- McIsaac, R. S. et al. Directed evolution of a far-red fluorescent rhodopsin. Proc. Natl Acad. Sci. USA111, 13034–13039 (2014). ArticleCASPubMedGoogle Scholar
- Jespers, L. S., Roberts, A., Mahler, S. M., Winter, G. & Hoogenboom, H. R. Guiding the selection of human-antibodies from phage display repertoires to a single epitope of an antigen. Biotechnology12, 899–903 (1994). CASPubMedGoogle Scholar
- Lai, Y. P., Huang, J., Wang, L. F., Li, J. & Wu, Z. R. A new approach to random mutagenesis in vitro. Biotechnol. Bioeng.86, 622–627 (2004). ArticleCASPubMedGoogle Scholar
- Myers, R. M., Lerman, L. S. & Maniatis, T. A general method for saturation mutagenesis of cloned DNA fragments. Science229, 242–247 (1985). ArticleCASPubMedGoogle Scholar
- Freese, E. Specific mutagenic effect of base analogues on Phage-T4. J. Mol. Biol.1, 87–105 (1959). ArticleCASGoogle Scholar
- Bridges, B. A. & Woodgate, R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc. Natl Acad. Sci. USA82, 4193–4197 (1985). ArticleCASPubMedGoogle Scholar
- Cox, E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu. Rev. Genet.10, 135–156 (1976). ArticleCASPubMedGoogle Scholar
- Greener, A., Callahan, M. & Jerpseth, B. An efficient random mutagenesis technique using an E. coli mutator strain. Mol. Biotechnol.7, 189–195 (1997). ArticleCASPubMedGoogle Scholar
- Scheuermann, R., Tam, S., Burgers, P. M. J., Lu, C. & Echols, H. Identification of the ε-subunit of Escherichia coli DNA polymerase III holoenzyme as the dnaQ gene product: a fidelity subunit for DNA replication. Proc. Natl Acad. Sci. USA80, 7085–7089 (1983). ArticleCASPubMedGoogle Scholar
- Ravikumar, A., Arrieta, A. & Liu, C. C. An orthogonal DNA replication system in yeast. Nat. Chem. Biol.10, 175–177 (2014). ArticleCASPubMedGoogle Scholar
- Leung, D. W., Chen, E. & Goeddel, D. V. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique1, 11–15 (1989). Google Scholar
- Zaccolo, M., Williams, D. M., Brown, D. M. & Gherardi, E. An approach to random mutagenesis of DNA using mixtures of triphosphate derivatives of nucleoside analogues. J. Mol. Biol.255, 589–603 (1996). ArticleCASPubMedGoogle Scholar
- Eckert, K. A. & Kunkel, T. A. High fidelity DNA synthesis by the Thermus Aquaticus DNA polymerase. Nucleic Acids Res.18, 3739–3744 (1990). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gupta, R. D. & Tawfik, D. S. Directed enzyme evolution via small and effective neutral drift libraries. Nat. Methods5, 939–942 (2008). ArticleCASPubMedGoogle Scholar
- Cadwell, R. C. & Joyce, G. F. Randomization of genes by PCR mutagenesis. PCR Methods Appl.2, 28–33 (1992). This seminal study in optimizing the conditions for epPCR is a must-read for all scientists performing random mutagenesis.ArticleCASPubMedGoogle Scholar
- Vanhercke, T., Ampe, C., Tirry, L. & Denolf, P. Reducing mutational bias in random protein libraries. Anal. Biochem.339, 9–14 (2005). ArticleCASPubMedGoogle Scholar
- Wong, T. S., Tee, K. L., Hauer, B. & Schwaneberg, U. Sequence saturation mutagenesis (SeSaM): a novel method for directed evolution. Nucleic Acids Res.32, e26 (2004). ArticleCASPubMedPubMed CentralGoogle Scholar
- Wells, J. A., Vasser, M. & Powers, D. B. Cassette mutagenesis: an efficient method for generation of multiple mutations at defined sites. Gene34, 315–323 (1985). ArticleCASPubMedGoogle Scholar
- Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods6, 343–341 (2009). ArticleCASPubMedGoogle Scholar
- Quan, J. Y. & Tian, J. D. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE4, e6441 (2009). ArticleCASPubMedPubMed CentralGoogle Scholar
- Nour-Eldin, H. H., Geu-Flores, F. & Halkier, B. A. User cloning and user fusion: the ideal cloning techniques for small and big laboratories. Methods Mol. Biol.643, 185–200 (2010). ArticleCASPubMedGoogle Scholar
- Reidhaarolson, J. F. & Sauer, R. T. Combinatorial cassette mutagenesis as a probe of the informational content of protein sequences. Science241, 53–57 (1988). ArticleCASGoogle Scholar
- Lehmann, M., Pasamontes, L., Lassen, S. F. & Wyss, M. The consensus concept for thermostability engineering of proteins. Biochim. Biophys. Acta1543, 408–415 (2000). ArticleCASPubMedGoogle Scholar
- Chen, F. et al. Reconstructed evolutionary adaptive paths give polymerases accepting reversible terminators for sequencing and SNP detection. Proc. Natl Acad. Sci. USA107, 1948–1953 (2010). ArticleCASPubMedGoogle Scholar
- Cherny, I. et al. Engineering V-type nerve agents detoxifying enzymes using computationally focused libraries. ACS Chem. Biol.8, 2394–2403 (2013). This paper nicely demonstrates how computational modelling can identify beneficial mutations, which can be stochastically incorporated into gene libraries.ArticleCASPubMedGoogle Scholar
- Das, R. & Baker, D. Macromolecular modeling with rosetta. Annu. Rev. Biochem.77, 363–382 (2008). ArticleCASPubMedGoogle Scholar
- Wijma, H. J. et al. Computationally designed libraries for rapid enzyme stabilization. Protein Eng. Des. Sel.27, 49–58 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Herman, A. & Tawfik, D. S. Incorporating synthetic oligonucleotides via gene reassembly (ISOR): a versatile tool for generating targeted libraries. Protein Eng. Des. Sel.20, 219–226 (2007). ArticleCASPubMedGoogle Scholar
- Stemmer, W. P. Rapid evolution of a protein in vitro by DNA shuffling. Nature370, 389–391 (1994). This study is the first to establish a method for homologous recombination of evolving protein populations.ArticleCASPubMedGoogle Scholar
- Coco, W. M. et al. DNA shuffling method for generating highly recombined genes and evolved enzymes. Nat. Biotechnol.19, 354–359 (2001). ArticleCASPubMedGoogle Scholar
- Muller, K. M. et al. Nucleotide exchange and excision technology (NExT) DNA shuffling: a robust method for DNA fragmentation and directed evolution. Nucleic Acids Res.33, e117 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Zhao, H., Giver, L., Shao, Z., Affholter, J. A. & Arnold, F. H. Molecular evolution by staggered extension process (StEP) in vitro recombination. Nat. Biotechnol.16, 258–261 (1998). ArticleCASPubMedGoogle Scholar
- Stemmer, W. P., Crameri, A., Ha, K. D., Brennan, T. M. & Heyneker, H. L. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene164, 49–53 (1995). ArticleCASPubMedGoogle Scholar
- Ness, J. E. et al. Synthetic shuffling expands functional protein diversity by allowing amino acids to recombine independently. Nat. Biotechnol.20, 1251–1255 (2002). ArticleCASPubMedGoogle Scholar
- Zha, D. X., Eipper, A. & Reetz, M. T. Assembly of designed oligonucleotides as an efficient method for gene recombination: a new tool in directed evolution. Chembiochem4, 34–39 (2003). ArticleCASPubMedGoogle Scholar
- Crameri, A., Whitehorn, E. A., Tate, E. & Stemmer, W. P. C. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat. Biotechnol.14, 315–319 (1996). ArticleCASPubMedGoogle Scholar
- Crameri, A., Raillard, S. A., Bermudez, E. & Stemmer, W. P. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature391, 288–291 (1998). ArticleCASPubMedGoogle Scholar
- Romanini, D. W., Peralta-Yahya, P., Mondol, V. & Cornish, V. W. A. Heritable recombination system for synthetic Darwinian evolution in yeast. ACS Synth. Biol.1, 602–609 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Sieber, V., Martinez, C. A. & Arnold, F. H. Libraries of hybrid proteins from distantly related sequences. Nat. Biotechnol.19, 456–460 (2001). ArticleCASPubMedGoogle Scholar
- Ostermeier, M., Shim, J. H. & Benkovic, S. J. A combinatorial approach to hybrid enzymes independent of DNA homology. Nat. Biotechnol.17, 1205–1209 (1999). ArticleCASPubMedGoogle Scholar
- Bittker, J. A., Le, B. V., Liu, J. M. & Liu, D. R. Directed evolution of protein enzymes using nonhomologous random recombination. Proc. Natl Acad. Sci. USA101, 7011–7016 (2004). ArticleCASPubMedGoogle Scholar
- Voigt, C. A., Martinez, C., Wang, Z. G., Mayo, S. L. & Arnold, F. H. Protein building blocks preserved by recombination. Nat. Struct. Biol.9, 553–558 (2002). CASPubMedGoogle Scholar
- Hiraga, K. & Arnold, F. H. General method for sequence-independent site-directed chimeragenesis. J. Mol. Biol.330, 287–296 (2003). ArticleCASPubMedGoogle Scholar
- Kolkman, J. A. & Stemmer, W. P. C. Directed evolution of proteins by exon shuffling. Nat. Biotechnol.19, 423–428 (2001). ArticleCASPubMedGoogle Scholar
- Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K. & Pease, L. R. Engineering hybrid genes without the use of restriction enzymes: gene-splicing by overlap extension. Gene77, 61–68 (1989). ArticleCASPubMedGoogle Scholar
- Gillam, E. M. J. Directed Evolution Library Creation (Springer, 2014). This book is an excellent resource for comparing and choosing between genetic diversification methods as well as for successfully executing library generation protocols.Google Scholar
- You, L. & Arnold, F. H. Directed evolution of subtilisin E in Bacillus subtilis to enhance total activity in aqueous dimethylformamide. Protein Eng.9, 77–83 (1996). ArticleCASPubMedGoogle Scholar
- Heim, R., Prasher, D. C. & Tsien, R. Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl Acad. Sci. USA91, 12501–12504 (1994). ArticleCASPubMedGoogle Scholar
- Kralj, J. M., Douglass, A. D., Hochbaum, D. R., Maclaurin, D. & Cohen, A. E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat. Methods9, 90–130 (2012). ArticleCASGoogle Scholar
- Cali, J. J. et al. Luminogenic cytochrome P450 assays. Expert Opin. Drug Metab. Toxicol.2, 629–645 (2006). ArticleCASPubMedGoogle Scholar
- Ostafe, R., Prodanovic, R., Lloyd Ung, W., Weitz, D. A. & Fischer, R. A high-throughput cellulase screening system based on droplet microfluidics. Biomicrofluidics8, 041102 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Gupta, R. D. et al. Directed evolution of hydrolases for prevention of G-type nerve agent intoxication. Nat. Chem. Biol.7, 120–125 (2011). ArticleCASPubMedGoogle Scholar
- Giger, L. et al. Evolution of a designed retro-aldolase leads to complete active site remodeling. Nat. Chem. Biol.9, 494–498 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Goddard, J. P. & Reymond, J. L. Enzyme assays for high-throughput screening. Curr. Opin. Biotechnol.15, 314–322 (2004). ArticleCASPubMedGoogle Scholar
- Fields, S. & Song, O. K. A novel genetic system to detect protein–protein interactions. Nature340, 245–246 (1989). ArticleCASPubMedGoogle Scholar
- Baker, K. et al. Chemical complementation: a reaction-independent genetic assay for enzyme catalysis. Proc. Natl Acad. Sci. USA99, 16537–16542 (2002). ArticleCASPubMedGoogle Scholar
- Lin, H. N., Tao, H. Y. & Cornish, V. W. Directed evolution of a glycosynthase via chemical complementation. J. Am. Chem. Soc.126, 15051–15059 (2004). ArticleCASPubMedGoogle Scholar
- Peralta-Yahya, P., Carter, B. T., Lin, H. N., Tao, H. Y. & Comish, V. W. High-throughput selection for cellulase catalysts using chemical complementation. J. Am. Chem. Soc.130, 17446–17452 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Swe, P. M. et al. Targeted mutagenesis of the Vibrio fischeri flavin reductase FRase I to improve activation of the anticancer prodrug CB1954. Biochem. Pharmacol.84, 775–783 (2012). ArticleCASPubMedGoogle Scholar
- Sengupta, D., Lin, H. N., Goldberg, S. D., Mahal, J. J. & Cornish, V. W. Correlation between catalytic efficiency and the transcription read-out in chemical complementation: a general assay for enzyme catalysis. Biochemistry43, 3570–3581 (2004). ArticleCASPubMedGoogle Scholar
- Fulwyler, M. J. Electronic separation of biological cells by volume. Science150, 910–911 (1965). ArticleCASPubMedGoogle Scholar
- Shapiro, H. M. Practical Flow Cytometry (Wiley-Liss, 2003). BookGoogle Scholar
- Boder, E. T. & Wittrup, K. D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol.15, 553–557 (1997). This paper describes the invention of yeast display protein libraries for screening protein–protein interactions and serves as the foundation for many other cell surface display methods.ArticleCASPubMedGoogle Scholar
- Santoro, S. W. & Schultz, P. G. Directed evolution of the site specificity of Cre recombinase. Proc. Natl Acad. Sci. USA99, 4185–4190 (2002). ArticleCASPubMedGoogle Scholar
- Wang, J. D., Herman, C., Tipton, K. A., Gross, C. A. & Weissman, J. S. Directed evolution of substrate-optimized GroEL/S chaperonins. Cell111, 1027–1039 (2002). ArticleCASPubMedGoogle Scholar
- Peck, S. H., Chen, I. & Liu, D. R. Directed evolution of a small-molecule-triggered intein with improved splicing properties in mammalian cells. Chem. Biol.18, 619–630 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Rajpal, A. et al. A general method for greatly improving the affinity of antibodies by using combinatorial libraries. Proc. Natl Acad. Sci. USA102, 8466–8471 (2005). ArticleCASPubMedGoogle Scholar
- Wang, X. X., Cho, Y. K. & Shusta, E. V. Mining a yeast library for brain endothelial cell-binding antibodies. Nat. Methods4, 143–145 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Chen, I., Dorr, B. M. & Liu, D. R. A general strategy for the evolution of bond-forming enzymes using yeast display. Proc. Natl Acad. Sci. USA108, 11399–11404 (2011). ArticleCASPubMedGoogle Scholar
- Qu, Z. et al. Immobilization of actively thromboresistant assemblies on sterile blood-contacting surfaces. Adv. Healthc. Mater.3, 30–35 (2014). ArticleCASPubMedGoogle Scholar
- Shi, J. H. et al. Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes. Proc. Natl Acad. Sci. USA111, 10131–10136 (2014). ArticleCASPubMedGoogle Scholar
- Ling, J. J., Policarpo, R. L., Rabideau, A. E., Liao, X. & Pentelute, B. L. Protein thioester synthesis enabled by sortase. J. Am. Chem. Soc.134, 10749–10752 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- McCluskey, A. J. & Collier, R. J. Receptor-directed chimeric toxins created by sortase-mediated protein fusion. Mol. Cancer Ther.12, 2273–2281 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Policarpo, R. L. et al. Flow-based enzymatic ligation by sortase A. Angew. Chem. Int. Ed Engl.53, 9203–9208 (2014). ArticleCASPubMedGoogle Scholar
- Swee, L. K., Lourido, S., Bell, G. W., Ingram, J. R. & Ploegh, H. L. One-step enzymatic modification of the cell surface redirects cellular cytotoxicity and parasite tropism. ACS Chem. Biol. (2014).
- Dorr, B. M., Ham, H. O., An, C., Chaikof, E. L. & Liu, D. R. Reprogramming the specificity of sortase enzymes. Proc. Natl Acad. Sci. USA111, 13343–13348 (2014). ArticleCASPubMedGoogle Scholar
- Yi, L. et al. Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries. Proc. Natl Acad. Sci. USA110, 7229–7234 (2013). ArticleCASPubMedGoogle Scholar
- Tawfik, D. S. & Griffiths, A. D. Man-made cell-like compartments for molecular evolution. Nat. Biotechnol.16, 652–656 (1998). The authors of this paper developed IVC as a platform for directed evolution. This study describes a selection for methyltransferases within water–oil emulsion droplets.ArticleCASPubMedGoogle Scholar
- Bernath, K. et al. In vitro compartmentalization by double emulsions: sorting and gene enrichment by fluorescence activated cell sorting. Anal. Biochem.325, 151–157 (2004). ArticleCASPubMedGoogle Scholar
- Agresti, J. J. et al. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl Acad. Sci. USA107, 4004–4009 (2010). ArticleCASPubMedGoogle Scholar
- Scott, D. J. & Plückthun, A. Direct molecular evolution of detergent-stable G protein-coupled receptors using polymer encapsulated cells. J. Mol. Biol.425, 662–677 (2013). ArticleCASPubMedGoogle Scholar
- Fischlechner, M. et al. Evolution of enzyme catalysts caged in biomimetic gel-shell beads. Nat. Chem.6, 791–796 (2014). In this study, polyelectrolyte shells served asin vitrocompartments for screening by flow cytometry.ArticleCASPubMedGoogle Scholar
- Bessette, P. H., Rice, J. J. & Daugherty, P. S. Rapid isolation of high-affinity protein binding peptides using bacterial display. Protein Eng. Des. Sel.17, 731–739 (2004). ArticleCASPubMedGoogle Scholar
- Mccafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature348, 552–554 (1990). In this pioneering study, phage display is demonstrated as a powerful technique to select high-affinity antibody fragments. This paper also nicely illustrates the guiding principles of related binding enrichments.ArticleCASPubMedGoogle Scholar
- Clackson, T., Hoogenboom, H. R., Griffiths, A. D. & Winter, G. Making antibody fragments using phage display libraries. Nature352, 624–628 (1991). ArticleCASPubMedGoogle Scholar
- Scott, J. K. & Smith, G. P. Searching for peptide ligands with an epitope library. Science249, 386–390 (1990). ArticleCASPubMedGoogle Scholar
- Becker, D. M. & Guarente, L. High-efficiency transformation of yeast by electroporation. Methods Enzymol.194, 182–187 (1991). ArticleCASPubMedGoogle Scholar
- Dower, W. J., Miller, J. F. & Ragsdale, C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res.16, 6127–6145 (1988). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hanes, J. & Pluckthun, A. In vitro selection and evolution of functional proteins by using ribosome display. Proc. Natl Acad. Sci. USA94, 4937–4942 (1997). ArticleCASPubMedGoogle Scholar
- Wilson, D. S., Keefe, A. D. & Szostak, J. W. The use of mRNA display to select high-affinity protein-binding peptides. Proc. Natl Acad. Sci. USA98, 3750–3755 (2001). ArticleCASPubMedGoogle Scholar
- Amstutz, P. et al. In vitro selection for catalytic activity with ribosome display. J. Am. Chem. Soc.124, 9396–9403 (2002). ArticleCASPubMedGoogle Scholar
- Seelig, B. & Szostak, J. W. Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature448, 828–831 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Orencia, M. C., Yoon, J. S., Ness, J. E., Stemmer, W. P. & Stevens, R. C. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat. Struct. Biol.8, 238–242 (2001). ArticleCASPubMedGoogle Scholar
- Palmer, A. C. & Kishony, R. Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nat. Rev. Genet.14, 243–248 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Liu, D. R., Magliery, T. J., Pasternak, M. & Schultz, P. G. Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc. Natl Acad. Sci. USA94, 10092–10097 (1997). This groundbreaking study on genetic code expansion exemplifies how selectable antibiotic resistance markers can form the basis for a range ofin vivoselections.ArticleCASPubMedGoogle Scholar
- Santoro, S. W., Wang, L., Herberich, B., King, D. S. & Schultz, P. G. An efficient system for the evolution of aminoacyl-tRNA synthetase specificity. Nat. Biotechnol.20, 1044–1048 (2002). ArticleCASPubMedGoogle Scholar
- Gaj, T., Mercer, A. C., Gersbach, C. A., Gordley, R. M. & Barbas, C. F. 3rd Structure-guided reprogramming of serine recombinase DNA sequence specificity. Proc. Natl Acad. Sci. USA108, 498–503 (2011). ArticlePubMedGoogle Scholar
- Young, E. M., Tong, A., Bui, H., Spofford, C. & Alper, H. S. Rewiring yeast sugar transporter preference through modifying a conserved protein motif. Proc. Natl Acad. Sci. USA111, 131–136 (2014). This study uses an auxotroph complementation strategy to select for sugar transporters that selectively uptake xylose from culture media.ArticleCASPubMedGoogle Scholar
- Lee, S. M., Jellison, T. & Alper, H. S. Directed evolution of xylose isomerase for improved xylose catabolism and fermentation in the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol.78, 5708–5716 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Worsdorfer, B., Woycechowsky, K. J. & Hilvert, D. Directed evolution of a protein container. Science331, 589–592 (2011). ArticleCASPubMedGoogle Scholar
- Takeuchi, R., Choi, M. & Stoddard, B. L. Redesign of extensive protein–DNA interfaces of meganucleases using iterative cycles of in vitro compartmentalization. Proc. Natl Acad. Sci. USA111, 4061–4066 (2014). ArticleCASPubMedGoogle Scholar
- Ghadessy, F. J., Ong, J. L. & Holliger, P. Directed evolution of polymerase function by compartmentalized self-replication. Proc. Natl Acad. Sci. USA98, 4552–4557 (2001). ArticleCASPubMedGoogle Scholar
- Ramsay, N. et al. CyDNA: synthesis and replication of highly Cy-dye substituted DNA by an evolved polymerase. J. Am. Chem. Soc.132, 5096–5104 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- d'Abbadie, M. et al. Molecular breeding of polymerases for amplification of ancient DNA. Nat. Biotechnol.25, 939–943 (2007). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ellefson, J. W. et al. Directed evolution of genetic parts and circuits by compartmentalized partnered replication. Nat. Biotechnol.32, 97–101 (2014). The authors of this paper evolved enzymes within IVCs by linking the desired phenotype to the expression of Taq polymerase withinE. coli. Taq can then be used in PCR to amplify the DNA encoding active library members within the emulsion droplet.ArticleCASPubMedGoogle Scholar
- Meyer, A. J., Ellefson, J. W. & Ellington, A. D. Directed evolution of a panel of orthogonal T7 RNA polymerase variants for in vivo or in vitro synthetic circuitry. ACS Synth. Biol.http:///dx.doi.org/10.1021/sb500299c (2014).
- Badran, A. H. & Liu, D. R. In vivo continuous directed evolution. Curr. Opin. Chem. Biol.24, 1–10 (2015). ArticleCASPubMedGoogle Scholar
- Lenski, R. E. & Travisano, M. Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc. Natl Acad. Sci. USA91, 6808–6814 (1994). ArticleCASPubMedGoogle Scholar
- Toprak, E. et al. Evolutionary paths to antibiotic resistance under dynamically sustained drug selection. Nat. Genet.44, 101–105 (2012). ArticleCASGoogle Scholar
- Muller, M. M. et al. Directed evolution of a model primordial enzyme provides insights into the development of the genetic code. PLoS Genet.9, e1003187 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Camps, M., Naukkarinen, J., Johnson, B. P. & Loeb, L. A. Targeted gene evolution in Escherichia coli using a highly error-prone DNA polymerase I. Proc. Natl Acad. Sci. USA100, 9727–9732 (2003). ArticleCASPubMedGoogle Scholar
- Bull, J. J. et al. Exceptional convergent evolution in a virus. Genetics147, 1497–1507 (1997). CASPubMedPubMed CentralGoogle Scholar
- Wichman, H. A., Wichman, J. & Bull, J. J. Adaptive molecular evolution for 13,000 phage generations: a possible arms race. Genetics170, 19–31 (2005). ArticleCASPubMedPubMed CentralGoogle Scholar
- Esvelt, K. M., Carlson, J. C. & Liu, D. R. A system for the continuous directed evolution of biomolecules. Nature472, 499–503 (2011). This study establishes a technological platform for the continuous evolution of biomolecules by linking the phage life cycle to the desired enzymatic activity.ArticleCASPubMedPubMed CentralGoogle Scholar
- Carlson, J. C., Badran, A. H., Guggiana-Nilo, D. A. & Liu, D. R. Negative selection and stringency modulation in phage-assisted continuous evolution. Nat. Chem. Biol.10, 216–222 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dickinson, B. C., Packer, M. S., Badran, A. H. & Liu, D. R. A system for the continuous directed evolution of proteases rapidly reveals drug-resistance mutations. Nat. Commun.5, 5352 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science319, 1387–1391 (2008). ArticleCASPubMedPubMed CentralGoogle Scholar
- Siegel, J. B. et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels–Alder reaction. Science329, 309–313 (2010). ArticleCASPubMedPubMed CentralGoogle Scholar
- Joh, N. H. et al. De novo design of a transmembrane Zn 2+ -transporting four-helix bundle. Science346, 1520–1524 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- King, N. P. et al. Accurate design of co-assembling multi-component protein nanomaterials. Nature510, 103–108 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Song, W. J. & Tezcan, F. A. A designed supramolecular protein assembly with in vivo enzymatic activity. Science346, 1525–1528 (2014). ArticleCASPubMedGoogle Scholar
- Karanicolas, J. et al. A de novo protein binding pair by computational design and directed evolution. Mol. Cell42, 250–260 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Patel, S. C. & Hecht, M. H. Directed evolution of the peroxidase activity of a de novo-designed protein. Protein Eng. Des. Sel.25, 445–452 (2012). ArticleCASPubMedGoogle Scholar
- Khersonsky, O. et al. Optimization of the in-silico-designed kemp eliminase KE70 by computational design and directed evolution. J. Mol. Biol.407, 391–412 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Rothlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature453, 190–195 (2008). This paper describes the computational design of a Kemp elimination catalyst. Subsequent screening yielded improved catalysts for a reaction that is not known to be performed by natural enzymes.ArticleCASPubMedGoogle Scholar
- Savile, C. K. et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science329, 305–309 (2010). ArticleCASPubMedGoogle Scholar
- Lutz, S. & Patrick, W. M. Novel methods for directed evolution of enzymes: quality, not quantity. Curr. Opin. Biotechnol.15, 291–297 (2004). ArticleCASPubMedGoogle Scholar
- Becker, S. et al. Single-cell high-throughput screening to identify enantioselective hydrolytic enzymes. Angew. Chem. Int. Ed Engl.47, 5085–5088 (2008). ArticleCASPubMedGoogle Scholar
- Lipovsek, D. et al. Selection of horseradish peroxidase variants with enhanced enantioselectivity by yeast surface display. Chem. Biol.14, 1176–1185 (2007). ArticleCASPubMedGoogle Scholar
- Piotukh, K. et al. Directed evolution of sortase A mutants with altered substrate selectivity profiles. J. Am. Chem. Soc.133, 17536–17539 (2011). ArticleCASPubMedGoogle Scholar
Acknowledgements
This work was supported by the US Defense Advanced Research Projects Agency grants DARPA HR0011-11-2-0003 and DARPA N66001-12-C-4207, the US National Institutes of Health (NIH)/National Institute of General Medical Sciences (NIGMS) (grant R01 GM095501) and the Howard Hughes Medical Institute (HHMI).









